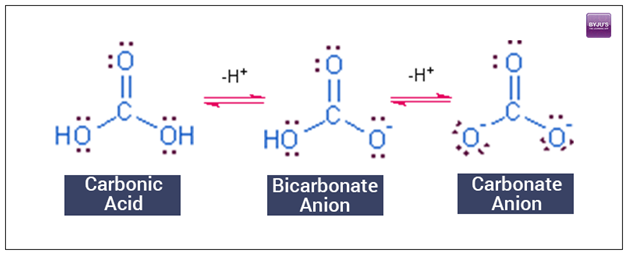
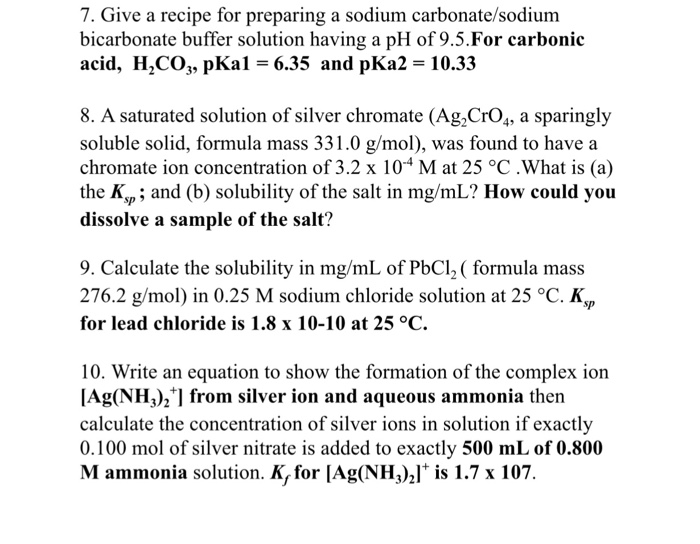
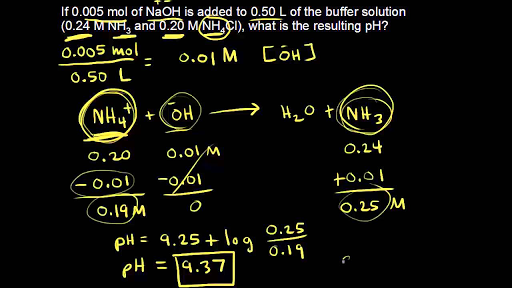
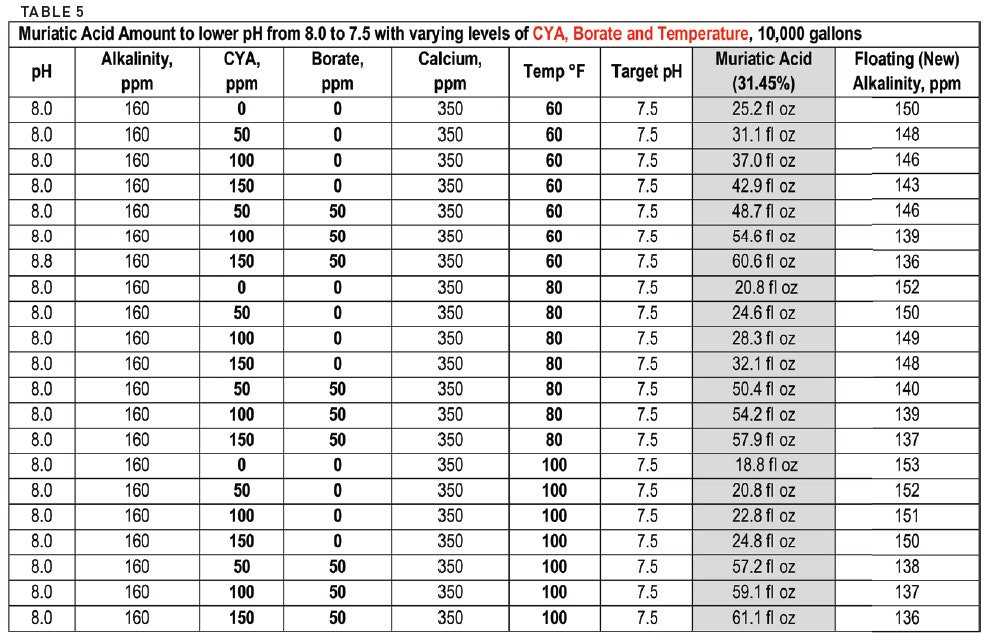
pH calculations and more in fundamentals of pharmaceutics. : Calculate pH and buffer capacity of a carbonic acid/ bicarbonate buffer solution.
![PDF] Calculation of the buffering capacity of bicarbonate in the rumen and in vitro. | Semantic Scholar PDF] Calculation of the buffering capacity of bicarbonate in the rumen and in vitro. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/104ba10db168516a4ba7d20867d92f2a51b891e7/3-Table1-1.png)
PDF] Calculation of the buffering capacity of bicarbonate in the rumen and in vitro. | Semantic Scholar
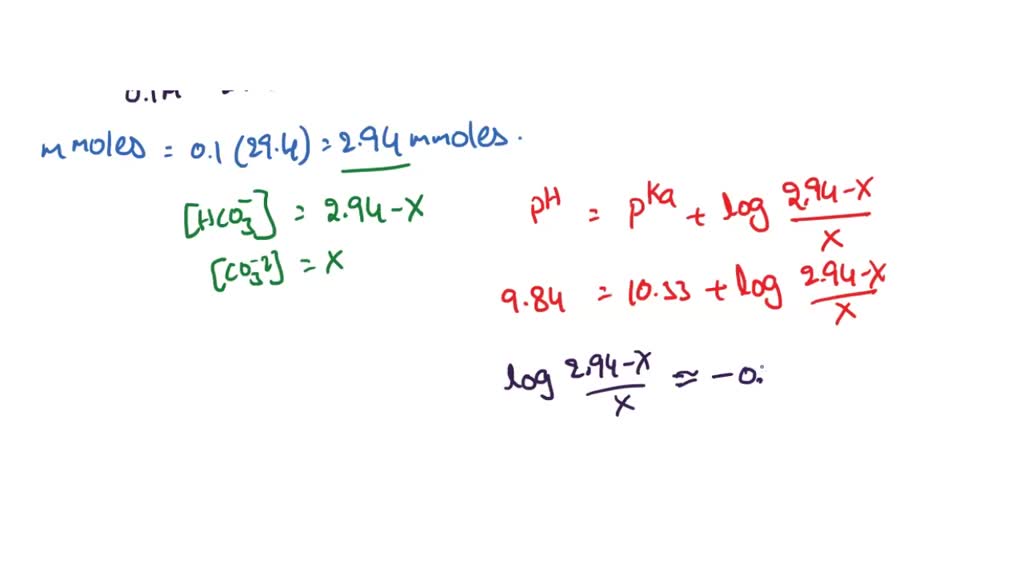



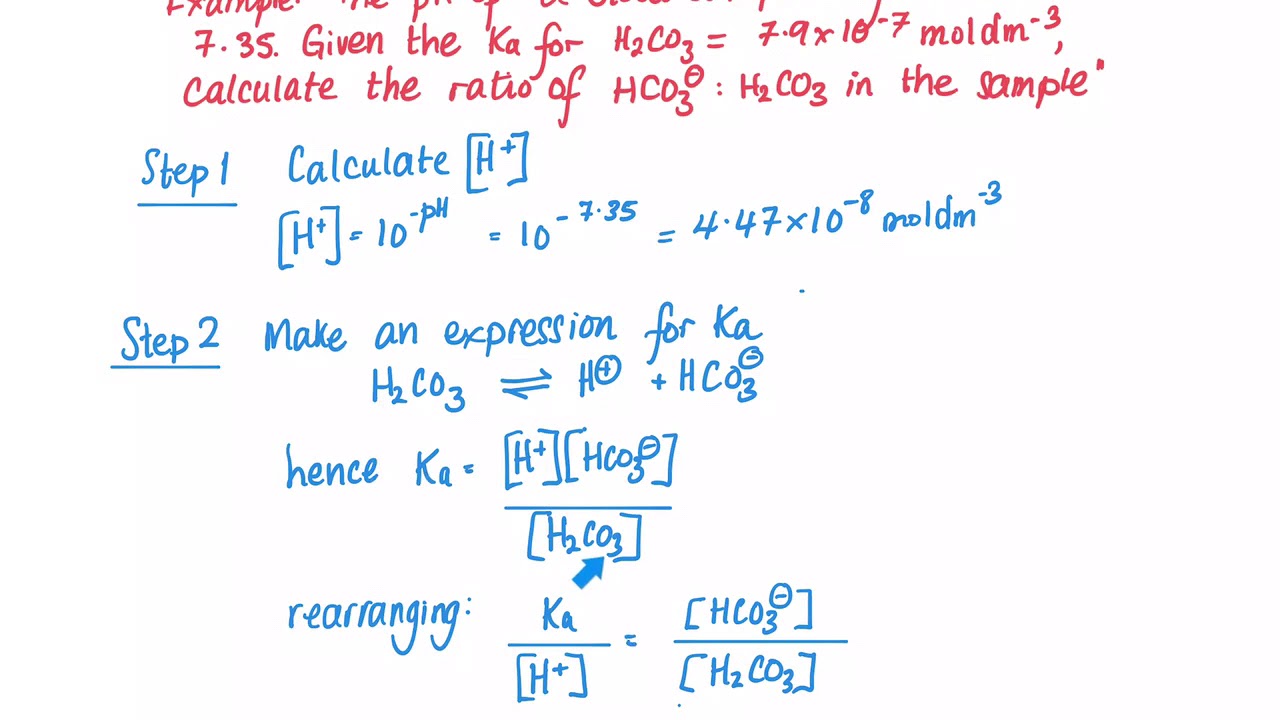
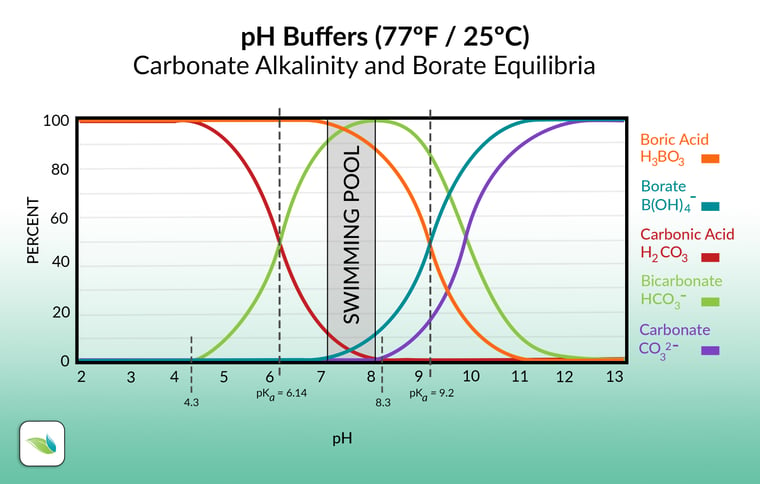

![PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/aaa98c1f9de4c398b6a057bf4936ea76736034c7/1-Table1-1.png)